Specific minerals/ions
The impact of carbonates on coffee flavour
Carbonates
Carbonate and bicarbonate ion are the most discussed anions when considering water composition because of it's role in alkalinity (acid buffering) and limescale in machines.
Unless you're using an espresso machine, you can ignore the latter.
High levels of carbonate can lead to a chalky, earthy taste (after all, calcium carbonate is chalk).
But don't worry too much – you would need very high acid buffer levels to give this taste, and our preference is for using a combination of citrate and bicarbonate
Carbonate's role as an acid buffer
In water, carbonate reacts with a single acid-produce H+ to become bicarbonate.
Carbonate's role in coffee water is to buffer the acids released from coffee, controlling (buffering!) the pH of the resulting brew and minimising the chance of an overly sour brew.
Here's what the buffering/pH activity of carbonate looks like:
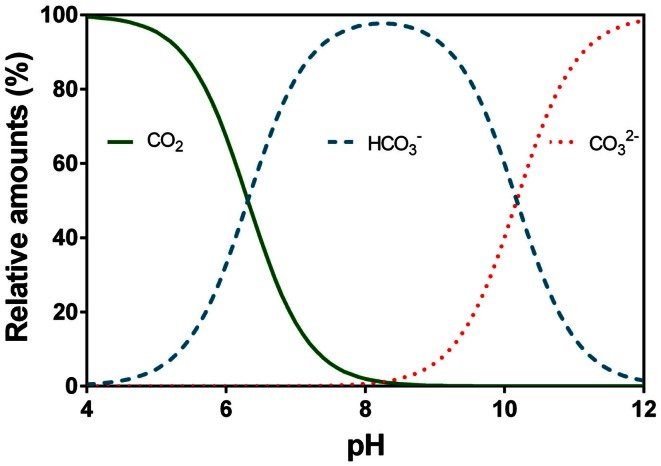
Coffee brews are normally around pH ~5, which is quite far past the mid-point of the HCO3 <> CO2 equilibrium.
This means as you brew the coffee > it releases acid > pH goes down, bicarbonate will turn into dissolved carbon dioxide.
That can stay dissolved in the water or leave as a gas.
This is why you may notice, when using a lot of carbonate/bicarbonate in your water, that you see more bubbling during a bloom, or slower filter times in some brewing methods.
The carbonate can react with acid from the coffee and release CO2, physically influencing the extraction.
The same issue arises in espresso, where under the heat/pressure/extraction, release of CO2 can interfere with the extraction itself.
The remaining carbonate is present as bicarbonate only -- unless you used a lot of sodium/potassium carbonate and have a pH >8 coffee, chances are you don't have any "carbonate" ion in the coffee, just bicarbonate.
Citrates
Traditionally, citrate has not been considered when looking at water alkalinity and coffee brewing.
But in recent years it has become more popular as an alternative buffering agent to carbonates.
Indeed coffee naturally releases citric, malic and tartaric acids, so it makes sense for a citrate acid buffer to be used – and it has other benefits.
Firstly, citrate and bicarbonate can be combined to provide a more complex buffering option, while also reducing the possibility of chalky taste from a lot of carbonates.
Citrate is more complex than bicarbonate having four (rather than 3) possible states, as it can react with 3 H+ from acid compared to two.
Here's what the equilibrium of these ions looks like with pH:
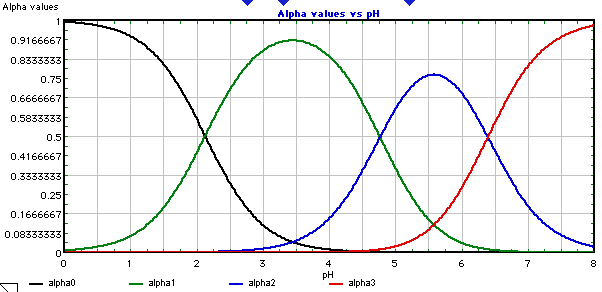
A few things to consider here:
Citrate buffers at lower pH than bicarbonate, so it will continue to buffer acidity at pH 5 better than bicarbonate will
Citrate does not transform into CO2, so will not create a gas that leaves the water and potentially impacts extraction
At pH 5, citrate actually exists as three different ions in solution, so likely gives more complexity in flavour
Because citric acid is naturally in fruits, coffee with fruity notes likely benefits from a citrate buffer more so than a carbonate one (though your own experiments will be more important here)
In our experience, a combination of citrate and bicarbonate is ideal with a target acid buffer concentration of 0.5-1.5 mM.
Using a combination, you can control acidity of the brew, while reducing the risk of any strong taste arising from the citrate or carbonates, respectively.